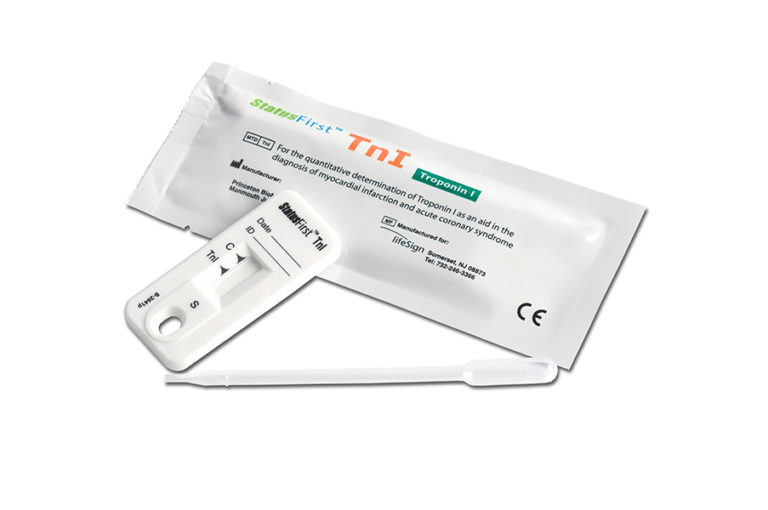
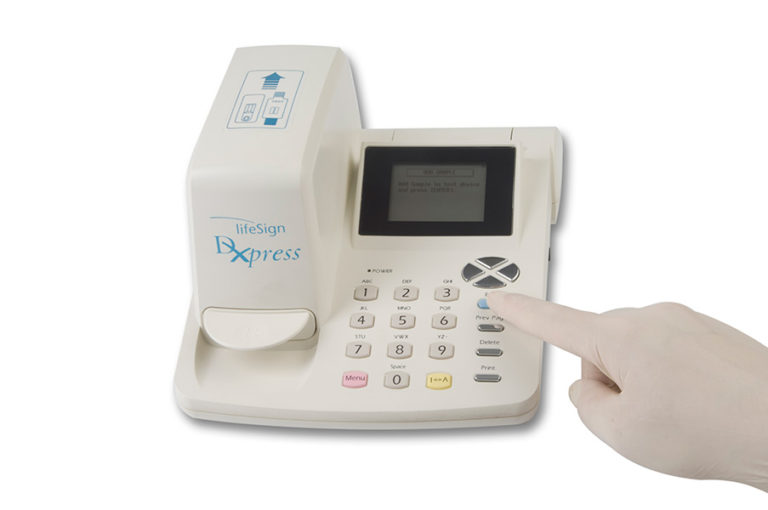
StatusFirst™ Troponin I Test
StatusFirst™TnI (Troponin I) is a rapid test for in vitro quantitative determination of Troponin I in human whole blood, plasma or serum. The device is intended for use with the DXpress Reader to provide quantitative results as an aid in the diagnosis of myocardial infarction and acute coronary syndrome (ACS). The StatusFirst TnI test device utilizes biotin coupled anti-TnI antibody/streptavidin solid phase chromatographic immunoassay technology to quantitatively determine the concentration of TnI in human whole blood, plasma, and serum specimens. After a sample has been dispensed into the sample well, the StatusFirst TnI test device is placed in the DXpress Reader. The DXpress Reader displays the TnI concentration 15 minutes after sample addition. The DXpress Reader is programmed to convert the intensity of the test band (as indicated by the “TnI” line on the test device) into a concentration of TnI automatically by using lot specific calibration factors supplied with each box of test devices. The TnI concentration in the sample correlates with the intensity of the test band.
Specification
Documents
Info Centre
Specification
Rapid on-site in vitro test for the detection of Troponin I
Device Format: Cassette (single use)
Sample Medium: Whole blood, serum or plasma
Sample Volume: 130 µL
Result Type: Quantitative
Result Interpretation: Using DXpress™ Reader
Result Time: 15 minutes
Shelf Life: 12 months from the date of manufacture
Storage Temperature: 2–8°C
Regulatory Approval: CE marked for professional use
Documents
Info Centre